Introduction
Chemical kinetics is a branch of chemistry that focuses on understanding the rates of chemical reactions and the factors influencing them. It provides insights into reaction mechanisms and plays a crucial role in industries, environmental studies, and everyday life. In this blog, we’ll explore the fundamentals of chemical kinetics, its importance, and how it impacts various fields.
What is Chemical Kinetics?
Chemical kinetics is the study of the speed or rate of chemical reactions and the steps involved in their progress. Unlike thermodynamics, which tells us whether a reaction will occur, kinetics explains how fast the reaction happens.
Key Concepts in Chemical Kinetics
Reaction Rate
The reaction rate measures how quickly reactants are converted into products. It is typically expressed as the change in concentration of a reactant or product per unit time.
Formula:
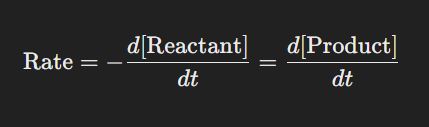
Rate Law
The rate law expresses the relationship between the rate of a reaction and the concentration of its reactants.
General form:
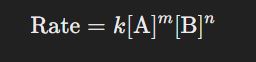
Order of Reaction
The sum of the powers of the reactant concentrations in the rate law. It can be zero, first, second, or higher.
Activation Energy (Ea)
The minimum energy required for reactants to form products. Lower activation energy leads to faster reactions.
Half-Life (t₁/₂)
The time required for half the concentration of a reactant to be consumed
Factors Affecting Reaction Rates
Concentration
Higher concentrations of reactants typically lead to faster reaction rates.
Temperature
Increasing temperature provides reactants with more kinetic energy, overcoming activation energy and speeding up reactions.
Catalysts
Catalysts lower the activation energy, increasing the reaction rate without being consumed in the process.
Surface Area
In heterogeneous reactions, a larger surface area of reactants enhances reaction rates.
Nature of Reactants
Ionic reactions are generally faster than covalent reactions because they involve less bond rearrangement.
Reaction Mechanisms
Chemical kinetics also investigates the step-by-step sequence of events in a reaction, known as the reaction mechanism. Each step may have its own rate, and the slowest step, called the rate-determining step, controls the overall reaction rate.
Example:
The decomposition of hydrogen peroxide (H2O2) involves an intermediate step that determines the overall reaction rate.
Applications of Chemical Kinetics
- Industrial Processes
- Optimizing reaction rates in processes like the Haber-Bosch method for ammonia production.
- Designing efficient catalysts for chemical industries.
- Environmental Chemistry
- Understanding pollutant degradation and the role of sunlight in atmospheric reactions.
- Modeling ozone layer depletion and greenhouse gas reactions.
- Pharmacokinetics
- Studying drug absorption, distribution, and metabolism to design effective medications.
- Food Industry
- Analyzing reaction rates to enhance food preservation techniques.
Conclusion
Chemical kinetics is a fascinating field that bridges theoretical concepts and practical applications. By understanding the factors influencing reaction rates and mechanisms, we can optimize processes, develop innovative solutions, and better comprehend natural phenomena.