Electrochemistry: Class 12 Notes, Summary, and Key Concepts
Electrochemistry, a vital topic in Class 12 Chemistry, focuses on the relationship between electricity and chemical reactions. This chapter not only lays the groundwork for advanced studies in chemistry but is also crucial for scoring well in board exams and competitive exams like CBSE Board NEET and JEE. Here’s a detailed guide to understanding electrochemistry with essential notes, formulas, and tips to ace your exams.
Table of Contents:
- Introduction to Electrochemistry
- Electrochemical Cells: Galvanic and Electrolytic
- Nernst Equation and Its Applications
- Conductance and Conductivity
- Electrolytic Conduction
- Batteries and Fuel Cells
- Corrosion: Causes and Prevention
- Important Formulas and Quick Tips for Exams
Introduction to Electrochemistry
Electrochemistry studies the interchange between chemical energy and electrical energy. It involves:
- Redox reactions: Where oxidation and reduction occur simultaneously.
- The flow of electrons between electrodes in an electrochemical system.
Key Applications:
- Batteries
- Electroplating
- Corrosion prevention
Electrochemical Cells: Galvanic and Electrolytic
Electrochemical cells are devices where chemical energy is converted into electrical energy or vice versa.
Galvanic Cells (Voltaic Cells):
Generate electricity from spontaneous redox reactions.
Example: Daniell Cell.
Electrode reactions:
- Anode (oxidation): Zn → Zn²⁺ + 2e⁻
- Cathode (reduction): Cu²⁺ + 2e⁻ → Cu
Nernst Equation and Its Applications
The Nernst Equation relates the electrode potential of a cell to the concentration of ions involved in the reaction.
Formula:
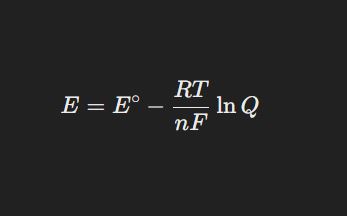
Applications:
- Calculating cell potential under non-standard conditions.
- Understanding concentration cells.
Conductance and Conductivity
Electrical conductance measures how easily electricity flows through a solution.
Key Terms:
- Conductance (G): Reciprocal of resistance. G=1RG = \frac{1}{R}G=R1
- Molar Conductivity (Λ): Conductivity of all ions in 1 mole of an electrolyte.
Kohlrausch’s Law:
At infinite dilution, the molar conductivity of an electrolyte is the sum of the individual contributions of its ions.
Electrolytic Conduction
Electrolytes conduct electricity by dissociating into ions in solution.
Types of Electrolytes:
Strong electrolytes: Fully ionize (e.g., HCl, NaCl).
Weak electrolytes: Partially ionize (e.g., CH₃COOH).
Factors Affecting Conductivity:
- Nature of electrolyte
- Concentration of solution
- Temperature
The Electrochemistry chapter in Class 12 Chemistry is fundamental for understanding the practical applications of redox reactions in everyday life. Be thorough with concepts like galvanic cells, electrolytic cells, and corrosion. Use diagrams, flowcharts, and solved examples for better clarity.
Stay consistent with your practice, and don’t forget to revise the Nernst Equation and formulas regularly. Share this guide with friends preparing for exams and stay tuned for more Class 12 Chemistry tips and resources!